
In the 1960s theoreticians tried to find a model which could be used to explain these hundreds of particles and the division into hadrons and leptons. Matter can be divided into hadrons (heavy particles) and leptons (light particles) By the early 1930s it was known that the nucleus consisted of positively charged protons and of neutrons, which have no electrical charge. This is the so called “solar system model” because of its similarity to our Solar System. Thus the modern picture of the atom emerged, negatively charged electrons in orbit around a positively charged nucleus.
#JJ THOMSON CATHODE RAY TUBE EXPERIMENT WITHOUT LABELS SERIES#
Then, in a series of experiments in 1909-10 the atomic nucleus was discovered by Ernest Rutherford and co-workes.

The first sub-atomic particle to be discovered was the electron, by J.J. The Periodic Table of the elements was drawn up in the mid 1800s, and by the end of the 19th Century scientists had measurements of the masses of different elements, noting that e.g. Through the work of John Dalton and others in the field of Chemistry, strong evidence was established that matter was composed of elementary building blocks, with each element being a different building block with different chemical properties. The idea of atoms thus dates back a couple of thousand years, but it was only in the 19th Century that evidence for their existence was really found. The word “atom” comes from the Greek word “ atomos” which means “indivisible”. The Universe is divided into matter, anti-matter and radiation. In replying to any such correspondence, he would insert random letter “p”s into the person’s name. One anecdote about Thomson is that he used to get annoyed when people would write to him and spell his name with the more traditional “p” in it, Thompson.
giving elements their chemical properties and forming bonds with other atoms.

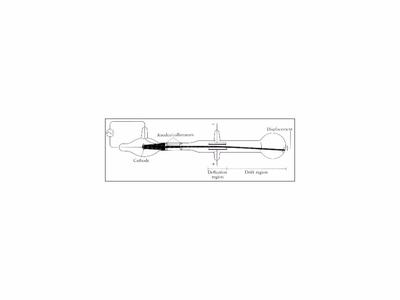
The electrons orbit the nucleus, but it is the electrons which are important in e.g. Cathode ray tubes were the basis for most TVs and computer monitors until this last 10 years, when more efficient Liquid Crystal Displays have largely replaced them.Īs I will describe in a future blog in more details, some 12-13 years after Thomson’s work, one of his ex-students Ernest Rutherford showed that most of the mass of an atom resides in the centre, in its nucleus. The very high voltage (thousands of volts) between the positive (anode) and negative (cathode) terminals causes electrons in the cathode to be accelerated to a high velocity as they are attracted towards the anode. Thomson thus showed that cathode rays are a stream of electrons. This of course indicated that what he had discovered was sub-atomic, a constituent of atoms. Thomson found that the mass of the particles he was deflecting in his magnetic and electric fields were thousands of times less massive than the mass of the lightest known element, Hydrogen. Carbon had a mass some 12 times the mass of Hydrogen.

By this time, chemists had fairly accurately determined the masses of atoms, and had shown that e.g. More than this, be was able to measure the shape of curve produced by the fields, and using the known strength of the fields he used, was able to calculate the mass and charge of the particles. The shadow of the Maltese cross appears on the glass. The green glow is produced by the charged electrons interacting with phosphorous in the glass, which then fluoresces. Electrons travel from the negative end (the cathode) towards the positive terminal (the anode).


 0 kommentar(er)
0 kommentar(er)
